The FDA approved Tuesday, Dec. 15, 2020, the marketing of the first over-the-counter home test of Covid-19, which can detect the presence of the virus within 20 minutes and sells for about $30.
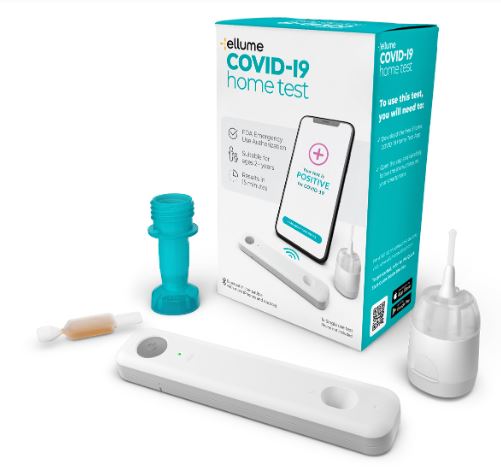
Ellume COVID-19 Test
The approval of this test by the U.S. Food and Drug Administration (FDA) is a “big step” in the fight against Covid-19, praised its head Stephen Hahn: “We’re helping Americans get access to more tests” and “reducing the burden on labs,” he added.
Results are available in about 20 minutes
The test is manufactured by California-based Ellume, which plans to make available three million units in January and millions more in the following months. The test uses a nasal swab that is not as long as those used in the medical field and therefore less painful. It must then be inserted into a small box connected via Bluetooth to the user’s smartphone.
The results are available in about 20 minutes in an application that must first be downloaded. The application asks the user for their zip code and date of birth in order to submit the data to health authorities. Providing your name and email address is optional.
Read Also: Do Adenovirus-Based COVID-19 Vaccines Increase the Risk of HIV Infection?
The test is an antigen test, which means it detects a molecule on the surface of the coronavirus. The technology used is similar to that of a pregnancy test. PCR tests, on the other hand, look for the genetic material of the virus.
Although antigen tests are less sensitive than PCR tests, many public health experts have been advocating their widespread use for months. They are inexpensive and could allow people to get tested as needed several times a week and get results almost immediately, while an ultra-precise test whose results are only known five or seven days later is useless, they argue because the status of the tested person may have changed by then
A fairly accurate test
According to the FDA, the Ellume test correctly identified 96% of positive samples and 100% of negative samples in symptomatic individuals. For individuals without symptoms, the test detected 91% of positive samples and 96% of negative samples.
Read Also: Messenger RNA Vaccines: How Do They Work and Are They Safe?
For patients without symptoms, the FDA recommends that positive results “be considered presumptive positive until confirmed by another test as soon as possible. Anyone with a positive result should be isolated, the agency said.
Ellume received $30 million in funding from the U.S. National Institutes of Health (NIH) to develop the test. Another rapid test, manufactured by Lucira Health, was approved by the FDA in mid-November but is available only by prescription.
References
Recommended Reads:
Coronavirus: The Real and False Side Effects of COVID-19 Vaccines
COVID-19: A List of the Vaccine Candidates Currently in Phase 3
Coronavirus Pandemic: The Flip-Flops of the Scientific Community
Is There A Link Between Pandemics and Climate Change?
Vaccines May Protect You Against Other Diseases Besides Those They Were Made for According To Study
Too Much Salt Weakens the Immune System According to Study
COVID-19: All the Essential Vitamins and Minerals for a Strong Immune System
FEEDBACK: